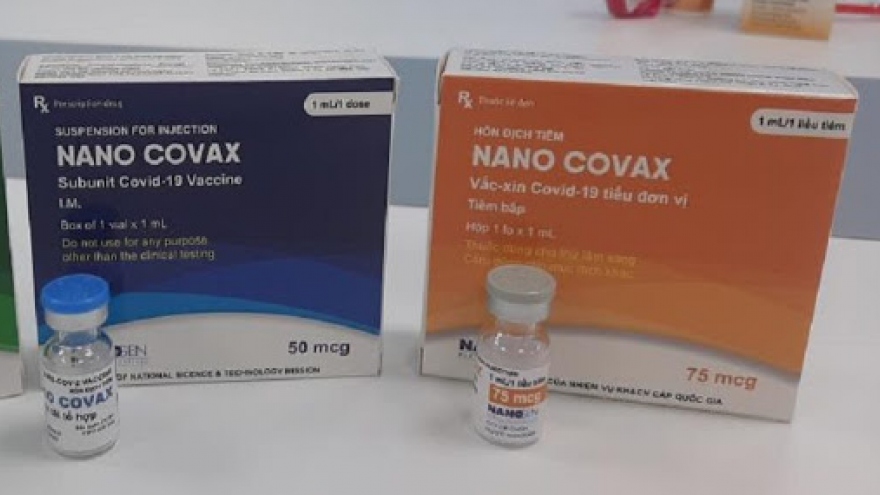
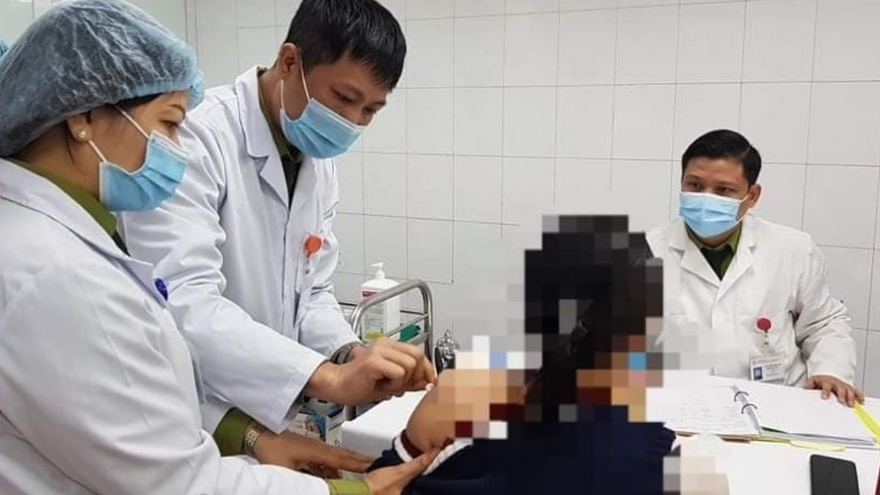
Volunteers receive second shots of Nano Covax in second-stage of human trials
VOV.VN - Up to 26 volunteers received their second shots of the Nano Covax novel coronavirus (COVID-19) vaccine on March 25 at the Vietnam Military Medical University in Hanoi as part of the second-stage of human trials to test the domestically-produced vaccine.
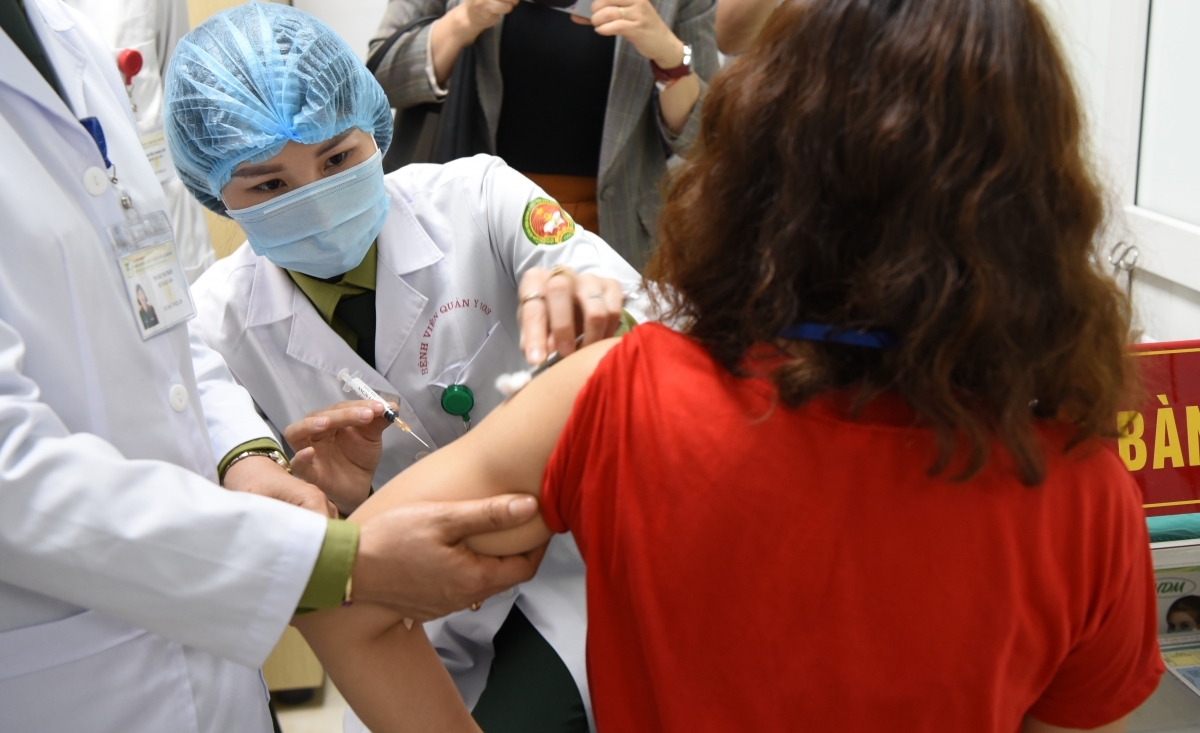
Ahead of the trials, all subjects had their health checked thoroughly before they were injected with the vaccine.
The second stage has been underway since February 26 and is being conducted by the university and the Ho Chi Minh City-based Pasteur Institute. Trials are being carried out at the university in Hanoi along with the medical centre of Ben Luc district in the southern province of Long An, with the participation of 560 volunteers aged between 18 and 60.
As part of the tests, volunteers were divided into four groups, with 80 people injected with a placebo whilst three other groups were administered with 25mcg, 50mcg, and 75mcg doses.
Among the subjects are 105 people aged over 60, with the eldest being 76 years old. Some volunteers also have some underlying health conditions which are not serious, such as high blood pressure, blood lipid disorders, diabetes, and cardiovascular diseases.
According to Chu Van Men, director of the Military Medical University’s Centre for Clinical Trials and Bioequivalence, the majority of the subjects remain in a stable condition after vaccination. Only a few recorded mild pain around the injection site and a fever that disappeared after only one to two days.
He added that the initial assessment indicates that the Navo Covax vaccine is “relatively safe” for volunteers.
The third phase of human trials is anticipated to cover between 10,000 and 15,000 people both locally and abroad. Providing that the results are positive, the nation will then administer the vaccine to the public in early 2022.
Furthermore, roughly 7,000 medical workers in Hanoi have received the Oxford/AstraZeneca COVID-19 vaccine since March 8.
Among those who have been vaccinated people, 33.21% of them have displayed normal side-effects. A total of 12 cases, accounting for 0.17% of subjects, experienced anaphylaxis, a reaction caused by severe allergy, of level one to three after vaccination, according to Chu Xuan Dung, vice chairman of Hanoi People’s Committee. Despite this, these cases remain in a stable condition, he added.
At present over 40,000 medical workers nationwide have been injected with the COVID-19 vaccine.